Introduction
Significant transformations have taken place in the field of cardiovascular medicine, largely driven by the remarkable advancements in echocardiography. Echocardiography has emerged as the second most widely used diagnostic test in cardiovascular medicine, following electrocardiography. This non-invasive technique enables the acquisition of crucial information regarding cardiac morphology, function, and hemodynamics. Over a span of less than fifty years, echocardiography has rapidly evolved into a fundamental technology in cardiovascular medicine.
The early stages of echocardiography's development witnessed groundbreaking milestones. The 1960s and 1970s marked the advent of the first academic course on cardiac ultrasound, the publication of the inaugural echocardiography textbook, and the introduction of the term "echocardiography" 1 itself. These significant contributions laid the foundation for the subsequent evolution of echocardiography.
Since its inception, echocardiography has undergone diverse advancements, resulting in the development of various types of this imaging technique.
The Origins of Echocardiography
Echocardiography has undergone a remarkable and significant improvement throughout its history. The origins of this technique can be traced back to the discovery of piezoelectricity in 1880, 2, 3 which led to the creation of ultrasound waves by piezoelectric crystals inside transducers.
Clinical echocardiography, as we know it today, emerged in the 1950s, thanks to the pioneering work of Carl Helmuth Hertz and Inge Edler. Edler, often referred to as the 'Father of Echocardiography,' made a breakthrough while assessing patients with mitral stenosis using the time motion or M-mode approach. 4 He identified a moving signal that corresponded to cardiac motion, and this technique was subsequently utilized for evaluating mitral stenosis. In 1954, 5 Edler and Hertz published their first paper titled 'The Use of Ultrasonic Reflectoscope for Continuous Movements of the Heart Wall.'
In 1969, Edler introduced the combined use of Doppler and echocardiography to diagnose aortic and mitral regurgitation. 6 The development of Doppler technology 7, 8 was pioneered by Japanese investigators. 9 Furthermore, in 1965, Harvey Feigenbaum and his colleagues reported the first detection of pericardial effusion using ultrasound.
Further advancements in echocardiography came with the introduction of the M-mode technique for measuring left ventricular dimensions, which was developed by Feigenbaum and Dodge in 1968. 10 Echocardiography gradually gained recognition as a highly valuable diagnostic technique, and after 1970, it became a regular feature in all annual meetings of the American College of Cardiology. 11 The first dedicated academic course on cardiac ultrasound took place in Indianapolis in 1968, and the first book on echocardiography was published in 1972.
Echocardiography's historical roots
Initially, Inge Edler referred to the technique as ultrasound cardiography (UCG). However, due to the widespread popularity of echoencephalography, which was the dominant examination for detecting echoes in the midline of the brain to assess intracranial space-occupying lesions, 11 the examination of the heart was named echocardiography. In the early years, the abbreviation "echo" couldn't be exclusively used since it couldn't differentiate between echocardiography and echoencephalography. Over time, as echoencephalography phased out as a diagnostic tool, echocardiography became the agreed-upon name for the cardiac examination. As of now, the abbreviation "echo" is commonly used to refer to echocardiography.
The Evolution of Various Types of Echocardiography
The field of cardiac ultrasound has witnessed numerous significant developments, many of which are too numerous to delve into in detail.
Transesophageal echocardiography (TEE) 12 was first described by an American individual. Japanese investigators also made contributions in this area, but TEE predominantly evolved into its current state through the efforts of European researchers. 13 It gained popularity after European investigators demonstrated its clinical utility.
Doppler technology has a similar story. The Japanese made a distinctive contribution with the development of color flow Doppler. However, the first paper on color flow Doppler was credited to the University of Washington in Seattle. 14 It was Hatle who demonstrated the accurate determination of hemodynamic data using Doppler echocardiography.
The journey of contrast echocardiography began at the University of Rochester when Gramiak and Shah accidentally observed the creation of large clouds of echoes within the heart after the injection of indocyanine green dye. 15 Contrast echocardiography has become an important diagnostic tool for visualizing left ventricular endocardial borders. The Mayo Clinic group reported the use of contrast echocardiography for detecting right-to-left shunts. Nowadays, commercial contrast agents are available that can pass through pulmonary capillaries and are visible on the left side of the heart.
Other advancements in echocardiography include three-dimensional echocardiography, intracardiac echo, myocardial velocity imaging, and 2D strain imaging, 16 which were developed sequentially.
The increased applications of echocardiography, coupled with the availability of portable machines, have made it even more prominent in clinical practice.17
Echocardiography in M-Mode
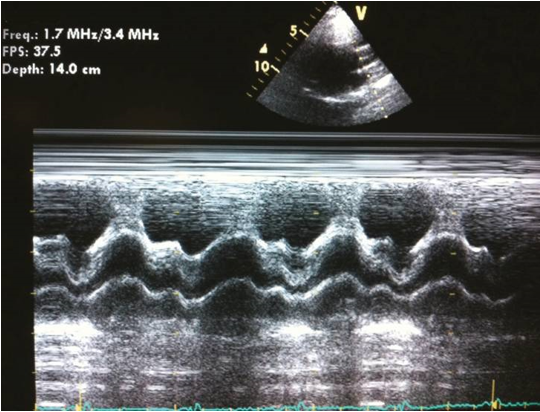
The initial form of cardiac ultrasound that was developed was M-mode echocardiography (Figure 1). In M-mode, a single ultrasonic beam is directed towards the heart, and the reflected signals are displayed on an oscillograph. 11 While M-mode echocardiography does not provide a detailed visualization of the actual anatomy of the heart, it serves a specific purpose in cases where precise measurements of cardiac time intervals during systole and diastole are required. It is also useful when studying rapidly moving targets such as vegetation. 11, 18
2-D Echocardiography
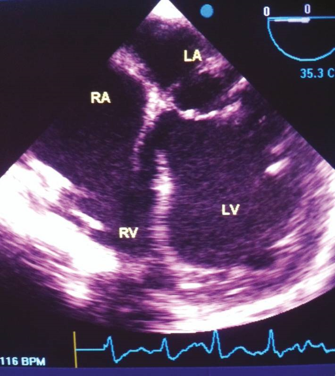
A significant breakthrough in the field of echocardiography occurred with the introduction of two-dimensional echocardiography, which revolutionized the ability to obtain real-time images of the heart. By sweeping an ultrasound beam back and forth through an arc, it became possible to create two-dimensional images of the heart’s 19 structures and function. Initially, two-dimensional images were reconstructed from M-mode tracings, thanks to the work of Gramiak. 20 Subsequently, the development of a popular real-time, two-dimensional scanner by Bom 21 further advanced the field. Additionally, Griffith and Henry 22 contributed to the progress by inventing a mechanical hand-held transducer device for two-dimensional scanning. The first successful and widely adopted commercial scanner was introduced by Eggleton and the Indiana group. Ever since, real-time, two-dimensional echocardiography has become the cornerstone of modern echocardiographic examinations. 11
Echocardiography with Doppler
Christian Doppler, an Austrian mathematician and physicist, made pioneering contributions to the understanding of the Doppler effect, 23 which involves the effect of an observer's motion relative to the source of an ultrasound wave. Despite Doppler's initial investigations, it took several decades for Doppler echocardiography to find clinical application, with its use becoming more prominent in the late 1970 24 In 1969, Doppler was first utilized to assess valvular regurgitation, marking an important milestone in its application to cardiology. Subsequently, researchers such as Holen and Hatle 25 demonstrated that the Doppler technique could provide accurate hemodynamic data, further solidifying its significance in cardiovascular assessment.
A significant breakthrough in Doppler ultrasound occurred in the 1970s with the successful quantification of pressure drops across valvular stenoses using the simplified Bernoulli equation. 26, 27 This discovery, made possible by Doppler ultrasound, provided a valuable tool for assessing the severity of stenotic valvular conditions. In the early 1970s, transesophageal Doppler allowed for the measurement of aortic blood flow velocity, while in the late 1980s, Doppler capabilities were incorporated into transesophageal probes, further enhancing their functionality. Another important development was the introduction of color-flow imaging in the early 1980s, which allowed for non-invasive visualization 28 of blood flow based on the Doppler principle. In 1982, Kitabatake and colleagues introduced pulsed-wave Doppler, enabling the recording of transmitral blood flow velocities and assessment of left ventricle diastolic function. This method has since become the primary clinical approach for non-invasive evaluation of diastolic filling patterns.
Echocardiography under Stress
In 1970, ultrasound was utilized to analyze left ventricular wall motion in healthy individuals both at rest and during exercise, providing valuable insights into cardiac function. Three years later, in 1973, M-mode echocardiography was employed to identify regional wall motion abnormalities (RWMA) 29 in the left ventricle. This technique proved useful in detecting abnormalities in specific areas of the heart's wall motion. In the late 1970s, the combination of exercise stress and M-mode echocardiography became a valuable tool for identifying wall motion abnormalities (WMA) 30 caused by ischemia. This approach allowed for the detection of impaired heart function during physical activity, indicating potential ischemic events. The advent of two-dimensional (2D) echocardiography sparked a particular interest in stress echocardiography. With the technological advancements and the development of digital echocardiography in the mid-1980s, which enabled the digital recording of both resting and stress images for side-by-side comparison, stress echocardiography became a routine clinical test. 31 Various methods were employed in early studies, including supine exercise, handgrips, upright bicycles, and cold pressor tests. 32 However, a significant advancement was made with the ability to record stress-induced wall motion abnormalities during treadmill exercise. Subsequently, pharmacological agents and cardiac pacing techniques were also employed to induce ischemic wall motion abnormalities for diagnostic purposes.
Cardiovascular transesophageal echography
In the 1970s, experimental probes were first introduced with the potential for transesophageal echocardiography (TEE). TEE, as we know it today, was initially performed in 1980 by placing a two-dimensional transducer on a fiberoptic endoscope. This marked the beginning of TEE's application in clinical practice. Subsequently, Hanrath and colleagues 33 made a significant advancement by attaching a phased-array ultrasound transducer to the tip of a flexible gastroscope, propelling TEE into its modern era. Initially, early monoplane transesophageal probes provided limited transverse images with a restricted field of view. However, with the development of smaller probes equipped with biplane and multiplane imaging capabilities, improved visualization of the heart became possible. This advancement greatly expanded the diagnostic capabilities of TEE. Being a semi-invasive procedure, TEE found utility in both inpatient and outpatient settings, allowing for progressive applications in the field of cardiology.
Intraoperative Echocardiography
Intraoperative echocardiography, performed through the epicardial or transesophageal approach, has become a valuable tool in cardiac surgery. Its use has evolved over time, starting with epicardial echocardiography in 1972 and later incorporating transesophageal echocardiography with color-flow imaging. 34
Before the initiation of cardiopulmonary bypass (CPB), intraoperative echocardiography aids in the assessment of cardiac structural and functional abnormalities. It helps surgeons identify any additional or previously overlooked findings that may necessitate a change in the surgical plan. 35, 36, 37
Following CPB, intraoperative echocardiography allows for the evaluation of surgical outcomes and the detection of new abnormalities that may require further intervention. By monitoring left ventricular (LV) function and identifying cardiovascular causes of hemodynamic instability during surgery, it can help reduce operative complications and difficulties encountered during off-pump procedures. 38, 39, 40
In hemodynamically unstable patients, intraoperative echocardiography aids in determining the underlying cause of compromised hemodynamics and identifying intraoperative complications. Additionally, it plays a crucial role in assessing the adequacy of valve repairs or replacements and surgical correction of congenital defects before the patient leaves the operating room. Its value is particularly well-established in patients undergoing mitral valve repair. 41, 42, 43
In 1999, the American Society of Echocardiography and Society of Cardiovascular Anesthesiologists published guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination. 44
Currently, intraoperative transesophageal echocardiography (IOTEE) is routinely requested for patients undergoing valve repair, those with aortic valve disease requiring valve replacement (to evaluate mitral regurgitation), myomectomy in patients with hypertrophic cardiomyopathy (HCM), cardiac mass removal, repair of intracardiac shunts (including atrial and ventricular septal defects), and all patients with congenital heart disease. 34, 35
Contrast Echocardiography
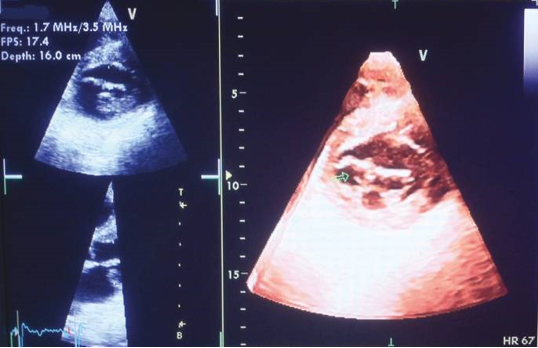
Contrast echocardiography has various clinical implications, particularly in the evaluation of intracardiac shunts. 45 Initially, agitated saline injection through a peripheral vein was utilized for this purpose. However, the inability to control the intensity of the contrast effect posed a significant challenge. To overcome this limitation, stable contrast agents were developed, specifically designed to opacify the right-sided cardiac chambers and assess intracardiac shunts. 46 This advancement resolved the problem of uncontrolled contrast intensity.
Subsequent studies demonstrated that intravenous left heart contrast agents not only enhance the definition of the left ventricular endocardial border but also improve image quality in patients with suboptimal image views (see Figure 3). 46, 47 In cases of ischemic heart disease, intravenous contrast agents can be employed to investigate myocardial perfusion. These agents offer valuable insights into the blood flow patterns within the myocardium.
Epicardial Echocardiography
In situations where transesophageal echocardiography (TEE) images are not optimal or when TEE is contraindicated, an alternative approach involves using a high-frequency ultrasound probe encased in a sterile sheath to visualize the heart from the epicardial surface. This technique allows for imaging of the heart by placing the probe directly on the outer surface of the heart, providing a different perspective and access to cardiac structures. By utilizing this method, healthcare professionals can obtain valuable information about the heart and its functioning, even when TEE is not feasible or ideal.
Three-Dimensional Echocardiography
The concept of three-dimensional (3D) echocardiography began to evolve during the 1960s, and the first reports of 3D scans of the heart were documented in 1974. 48 Initially, 3D echocardiograms were obtained using a technique called reconstruction. 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 This technique involved acquiring ECG-gated images from various transducer positions with precise locations. Each image was then placed in its appropriate three-dimensional spatial position within the cardiac cycle using specialized software programs. Through specific image processing techniques, the structure could be reconstructed as a 3D object, allowing for the visualization of surfaces and volumes.
Advancements in transthoracic transducers have made it possible to obtain real-time or near real-time 3D volume data sets. The development of more sophisticated transthoracic transducers has facilitated the acquisition of 3D images in a dynamic and immediate manner, enhancing the clinical utility of 3D echocardiography.
The field of 3D echocardiography has experienced rapid advancements, leading to the availability of various types of real-time 3D imaging techniques. Modern real-time 3D systems employ matrix array transducers equipped with 3000-4000 elements, allowing for detailed and high-resolution imaging.
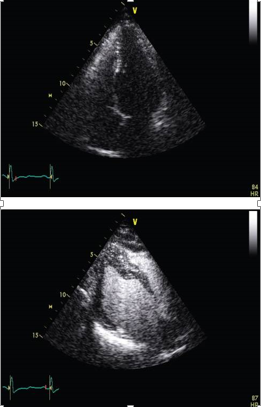
The ability of 3D echocardiography to depict cardiac structures in close approximation to their real forms has proven to be particularly beneficial in the evaluation of complex congenital heart disease (see Figure 4). This imaging modality provides clinicians with valuable insights into the intricate anatomical features and spatial relationships within the heart, enhancing the understanding and management of congenital heart conditions.
Figure 4
This figure depicts a transverse view at the level of the mitral valve obtained from the parasternal short axis using full-volume 3D imaging. It showcases a patient with prolapse of the posterior leaflet and a visible arrow indicating the presence of ruptured chordae.
Real-time 3D echocardiography has demonstrated high accuracy, feasibility, and value in the operating room, as well as in various clinical applications.49 Its utilization in contrast-enhanced imaging provides several advantages, particularly in improving the quantification of left ventricular volumes. 50 Moreover, the scope of clinical applications for 3D echocardiography is rapidly expanding. However, the most common indications for real-time 3D echocardiography remain quantitative measurements of left ventricular volumes, assessment of regional wall motion abnormalities (RWMA), congenital heart disease, valvular disease (as shown in Figure 4), and evaluation of ventricular desynchrony.
3D echocardiography offers several advantages over 2D echocardiography, leading to improved visualization of complex shapes and relationships between cardiac structures. It enables more accurate calculation of cardiac volumes, mass, and function, providing comprehensive information about the heart's performance. Additionally, 3D echocardiography allows for enhanced imaging of colour Doppler flow fields, enabling better assessment of blood flow patterns. It also facilitates the evaluation of valvular abnormalities and dysfunctions, offering a more comprehensive understanding of valve structure and function. Overall, 3D echocardiography presents numerous benefits for detailed cardiac assessment compared to traditional 2D echocardiography.
Tissue Doppler Imaging (TDI) and Strain and Strain Rate Imaging (SRI)
The Doppler technique is employed in measuring myocardial velocities due to the contrasting signal characteristics between blood and myocardium. There are notable differences in signal amplitudes and Doppler frequencies between these two components. While the speed of myocardial motion is considerably lower compared to blood flow, the amplitude of myocardial signals is significantly higher. These distinctions enable the implementation of filters that selectively isolate myocardial velocities while rejecting echo signals originating from the blood pool. The recording of velocities can be achieved using either color Doppler or pulsed wave Doppler techniques.
The introduction of myocardial velocity imaging dates back to the early 1990s, 51, 52 and since then, it has become a well-established method for quantifying both left ventricular (LV) systolic and diastolic function. Real-time imaging of myocardial motion allows for the visualization of color-coded velocities overlaid on a 2D grayscale image. The frame rate for 2D color Doppler typically ranges between 80 and 200 frames per second, depending on the sector width, and is usually set higher than that of simultaneous grayscale images. The analysis of myocardial velocities can be performed offline, as shown in Figure 5.
Figure 5
This figure presents offline analyses of tissue Doppler imaging performed on a healthy individual. The analyses were conducted from the apical 4-chamber view, focusing on the basal, middle, and apical segments of the interventricular septum.
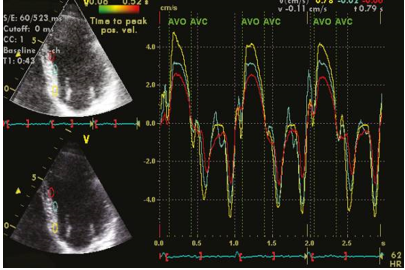
Clinical application of tissue Doppler velocity imaging extends to various areas. It can be utilized for diagnosing myocardial ischemia, assessing patients with diastolic dysfunction, and aiding in the selection of suitable candidates for cardiac resynchronization therapy through the evaluation of ventricular dyssynchrony, as depicted in Figure 6. Tissue Doppler velocity imaging plays a crucial role in these clinical scenarios, providing valuable information for diagnosis, evaluation, and treatment decisions.
Figure 6
This figure compares the assessment of left ventricular dyssynchrony using tissue Doppler imaging from the apical 4-chamber view in two scenarios. The right panel represents a normal patient, while the left panel represents a patient with left bundle branch block (LBBB). Dyssynchrony was determined by measuring the maximal differences in time-to-peak regional velocity between the septal and lateral walls.
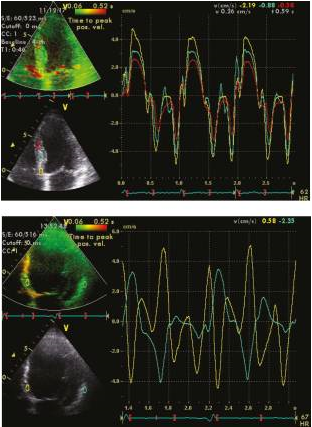
Speckle Tracking Echocardiography (STE)
Non-Doppler 2D strain imaging is an innovative technique used to measure strain and strain rate values. This method involves analyzing motion patterns by tracking natural acoustic markers known as speckles in 2D grayscale images. The speckles are formed due to the interference of ultrasound beams in the myocardium, and they serve as inherent acoustic markers that can be tracked frame by frame. This tracking process allows for the quantification of myocardial deformation and provides valuable insights into the mechanics and function of the heart. 53, 54 The use of non-Doppler 2D strain imaging represents a significant advancement in cardiac imaging techniques.
Speckle tracking echocardiography (STE) utilizes an automatic measurement technique that assesses the distance between speckles, enabling angle-independent strain measurement. This method allows for simultaneous measurements from multiple regions within an image plane, as depicted in Figure 7. In contrast, Doppler-based strain measurements rely on calculating velocities between a fixed point and a reference point, typically an external probe.
Figure 7
This figure illustrates the evaluation of left atrial (LA) strain and strain rate using velocity vector imaging. The maximum strain measurement was taken during left ventricular (LV) systole, while the strain rate measurement was obtained during the early phase of LV filling.
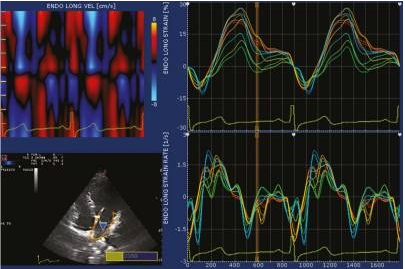
STE provides a direct measure of strain by analyzing the distance between two points within a specific region of the myocardium. In contrast, tissue Doppler imaging (TDI) calculates strain by integrating strain rate (SR) measurements. 55, 56, 57 The use of speckle tracking in STE offers advantages over TDI, as it allows for a more comprehensive and direct assessment of myocardial strain.
STE offers significant advantages, particularly its independence from ultrasound angle and translational motion of the heart within the chest. These features enable the measurement of circumferential and radial strains from the left ventricular (LV) short axis, as well as longitudinal strain from apical myocardial regions. Additionally, STE holds promise for measuring LV rotation and torsion, further enhancing its capabilities in assessing myocardial mechanics. 56, 57, 58
While higher frame rates are necessary for measuring peak velocities and strain rates, optimal frame rates of around 80 frames per second are typically sufficient. STE finds clinical application in evaluating regional ventricular and atrial myocardial function, as well as in assessing atrial and ventricular dyssynchrony. Its versatility in assessing various aspects of cardiac function makes it a valuable tool in clinical practice.
Velocity Vector Imaging (VVI) is an innovative imaging technique that utilizes routine 2D grayscale echo images to calculate and visualize regional motions in terms of velocity and direction. This technique combines speckle tracking, mitral annulus motion, and tissue-blood border detection to derive valuable information about myocardial motion patterns. By integrating these different components, VVI provides a comprehensive assessment of regional myocardial dynamics and offers a unique perspective on cardiac function. VVI represents a promising advancement in echocardiography, allowing for detailed analysis of regional myocardial motion using standard grayscale images.59
Intracardiac Echocardiography
In recent years, catheter-based probes with Doppler capabilities have been introduced in clinical practice, bringing forth significant advancements.60 One notable application is transcatheter intracardiac echocardiography (ICE), which plays a valuable role in guiding trans-catheter interventions, specifically atrial septal defect device closure. ICE has proven to be highly beneficial in providing real-time imaging guidance during these procedures.
Furthermore, ICE has demonstrated utility in electrophysiology procedures, such as pulmonary vein ablation for atrial fibrillation. It aids in the detection of complications like pulmonary vein stenosis and assists in the precise placement of multisite pacing catheters.
The integration of Doppler capabilities within catheter-based probes has expanded the possibilities of intracardiac echocardiography, enhancing its role in guiding various interventional and electrophysiology procedures. ICE has become an invaluable tool in improving the safety and effectiveness of these interventions.
Role of Echocardiography in the New Era of Medical Cost Containment
The rapid development of echocardiography presents both positive and negative aspects. On the positive side, echocardiography has advanced significantly, providing physicians with valuable tools for state-of-the-art examinations. However, this progress also means that physicians must put in continuous effort to stay up to date and proficient in utilizing these new techniques. Every new echocardiographic application requires a learning curve, demanding considerable time and dedication from physicians to become experts in these methods. 1
In the current era of cost containment, comprehensive and appropriate echocardiography has become a necessity. Not only is echocardiography relatively cost-effective, but it also has the potential to provide definitive information. By conducting thorough echocardiographic studies, the need for more expensive and potentially harmful examinations can be eliminated for the majority of patients. This approach has a significant impact on the cost-effectiveness of patients' care, ensuring that resources are allocated wisely while delivering high-quality diagnostic information.
Conclusion
Echocardiography holds a crucial role in cardiology practice, yet like any technology, it comes with both advantages and disadvantages. One significant disadvantage is the learning curve required to perform quantitative examinations and interpretations effectively. This underscores the need for physicians to invest time and effort in mastering the techniques and skills necessary for accurate and reliable echocardiographic assessments.
However, the principal advantage of echocardiography lies in its exceptional versatility as a diagnostic tool. It offers a wide range of imaging capabilities, allowing for detailed assessments of cardiac structure and function. When performed properly, with the appropriate patient selection and clinical indications, echocardiography can be highly cost-effective. By providing valuable diagnostic information, it helps guide appropriate management decisions, reducing the need for more invasive and costly procedures.
In summary, despite the learning curve associated with echocardiography, its versatility and the potential for cost-effectiveness make it an indispensable tool in cardiology practice. With proper utilization and interpretation, echocardiography plays a crucial role in providing valuable insights into cardiac health and optimizing patient care.