- Visibility 517 Views
- Downloads 83 Downloads
- DOI 10.18231/j.jchm.2022.030
-
CrossMark
- Citation
High dose radioactive iodine therapy and its short term adverse effects
- Author Details:
-
Ancy George *
-
Annapurna Y
-
Harilal P
-
Anila Kumari
Introduction
Disorders of thyroid glands are observed mostly in women, the reason is unknown.[1], [2] It is estimated that 42 million people in India are affected by thyroid disorders.[3] The prevalence of hyperthyroidism in the Western population is 2.5-4.7/1000 females,[4] which is comparable to the prevalence in the South Indian population of subclinical and overt hyperthyroidism being 1.6% and 1.3% respectively.[3]
Thyroid carcinoma
Thyroid carcinoma can be differentiated, poorly differentiated, or undifferentiated variants. Differentiated carcinoma of the thyroid gland can be papillary thyroid carcinoma and follicular thyroid carcinoma, the most common variant of thyroid carcinoma with a good prognosis,[5] accounting for 80-90% of the cases. Patients usually will be asymptomatic presenting with a thyroid nodule. There may be cases of cough, difficulty in swallowing, and breathing. History and clinical examination of thyroid swelling can be followed by thyroid function test tests, thyroid ultrasound scan, and FNAC to confirm the diagnosis.
The treatment modalities consist of different expertise. Surgery and radioactive iodine followed by levothyroxine remain the treatment of choice for differentiated thyroid cancers.[6] Surgical therapy- total thyroidectomy- is the cornerstone for all types.[7] It may be with or without concomitant central neck dissection according to the stage and cervical lymph node metastasis. Post-operative ablation therapy with radioactive iodine has been established as an adjunct therapy.[8] Radioactive iodine benefits the destruction of malignant cells.
Radio-active iodine (RAI-131)
It is used in medical therapies as a treatment and diagnostic tool. As iodine is naturally taken up in the thyroid gland, this mechanism is utilized by radioactive isotopes for entry and localization inside the gland. When administered, it emits beta rays and gamma rays. Beta rays kill overactive thyroid cells and gamma rays can affect the persons in the vicinity.[2]
Radioiodine ablation refers to the destruction of the residual macroscopically normal thyroid tissue after completing the gross surgical resection of cancer.[8] RAI collects mainly in thyroid cells and radiation kills the cancer cells with little effect on the rest of the body.[9] In the case of hyperthyroidism, radioactive iodine is taken up by the iodide transporter; the beta particles destroy the follicle cells leading to control of thyrotoxicosis symptoms.[10]
Mechanism
I-131 is used for the treatment and diagnosis of thyroid carcinoma and the treatment of hyperthyroidism. I-131 is taken inside the thyroid cells with the help of sodium/ iodide symporter. Ionizing radiation emitted leads to the destruction of thyroid cells which is its therapeutic effect. Beta radiation is responsible for this therapeutic action. The oral dosage form is available as sodium iodide when taken is absorbed and distributed in extracellular fluid. It is trapped by thyroid cells and exerts its action. I-131 decays by beta emission and the half-life is 8.04 days.[11] It is later eliminated by kidneys.
Radioactive iodine therapy (RAIT) is used in low, moderate, high, and very high doses. For hyperthyroidism, a low dose (<30 mCi) is usually used.[12] For DTC, the doses are moderate (31-50 mCi), high (51- 150 mCi), and very high (>150 mCi).[13]
Radioactive iodine has also been shown to decrease mortality and recurrence in high-risk patients and is important in the treatment of invasive or metastatic disease.[14]
Adverse effects
RAIT also has its adverse effects which can be classified as short term and long term adverse effects.[15] Long-term effects include increased risk of malignancy- leukemia and secondary cancers, radiation pulmonary fibrosis, permanent bone marrow suppression, genetic defects, chronic xerostomia. The short-term adverse effects, even though mild, are more frequent – which include gastritis- nausea and vomiting, radiation thyroiditis, sialadenitis/xerostomia, bone marrow depression, dry eye, and nasolacrimal obstruction.[16]
In males, RAIT may cause hypospermia and in females, it can cause complications in pregnancy and also ovarian complications.[14] But the female patients who had undergone radioiodine ablative therapy in childhood did not develop any abnormalities of their reproductive tracts later in life.[17]
Among the short-term adverse effects, gastrointestinal discomforts, salivary gland dysfunction and swelling of the neck is most common.[15] Gastrointestinal discomforts include nausea and vomiting and are more frequent in children in whom nausea is the rule.[18] Sialadenitis/ xerostomia is a common adverse effect seen with RAIT. Sialadenitis is salivary gland inflammation that leads to xerostomia.[19] It can also cause pain, tenderness, and bitter taste. Dry mouth can be managed pharmacologically as well as non-pharmacologically. The drugs used are pilocarpine and amifostine but are usually avoided due to their high side effects.[20] Non-pharmacological treatment includes administration of sialagogues – Vitamin C, Vitamin E, lemon juice, lemon candy, and chewing gum. Sialagogues administration can lead to decreased damage to the salivary gland.[20]
The different categories of the patient based on radioactive iodine dosing are:
Low dose (< 30 mCi)
Moderate dose (31-50 mCi)
High dose (50- 150 mCi)
Very high dose (>150mCi)[13]
The incidence of short-term adverse effects following RAIT is 44%,[15] of which gastritis accounts for 30%, sialadenitis/ xerostomia accounts for 30%, and radiation thyroiditis for 20%.[21] The first I-131 ablative therapy was shown to impair the salivary uptake and secretory function of patients with differentiated thyroid cancer (DTC).[22]
RAIT has also been shown to cause hematological changes. WBC and platelet decrease after 1 month of RAIT.[23] whereas RBC and hemoglobin showed a transient drop after 1 month. This study aims to identify the short-term adverse effects following radioactive iodine at high doses for DTC. Since identifying the adverse effects can help in better managing the patient which is worthwhile for his/her further course of treatment, this study is being done for the beneficence of the participants.
Objectives
To evaluate the pattern of short-term adverse effects following high doses of radioactive iodine therapy in patients with thyroid cancers.
Materials and Methods
Study population
All the patients undergoing radioactive iodine therapy at a high dose, that is, above 30mCi, for thyroid cancers fulfilling the inclusion criteria attending the Department of Nuclear Medicine, Government Medical College, Kozhikode.
Study design: Prospective observational study
Sample size: 75
Study setting: The study was approved by the Ethics Committee of Government Medical College, Kozhikode, and was conducted in the Department of Nuclear Medicine, Government Medical College, Kozhikode, and the Department of Pharmacology, Govt. Medical College, Kozhikode.
Study duration: The study was conducted for a period of one year from January 2019 to January 2020.
Inclusion criteria
Patients with DTC who treated with a dose above 30 mCi.
Patients giving written informed consent.
Exclusion criteria
Patients who had undergone treatment with a dose less than 30 mCi.
Known cases of hematological malignancies.
Patients not giving informed consent.
Informed consent
Written informed consent was taken from the patient before the study.
Study procedure
The study was started after obtaining scientific Research and Ethics committee approval. After screening, patients who fulfilled the inclusion criteria were enrolled in the study.
The demographic details of the patients were collected, followed by details regarding present RAIT including date, the dose of RAIT, TSH values, and diagnosis of the patient. Baseline complete blood routine values were also collected. Adverse effects following RAIT. Post - RAIT Blood routine values were also noted.
Outcome assessment
The pattern of short-term adverse effects following high dose of radioactive iodine therapy. The common short-term adverse effects include – gastritis, sialadenitis, xerostomia, neck pain, thyroiditis, and decreased total peripheral count. The severity of hematological abnormalities was assessed by blood routine examination.
Statistical Analysis
Statistical analysis was done using Statistical Package for Social Science (SPSS) software version 18. All the participants who underwent high dose radioiodine therapy were included in the statistical analysis. The outcome was expressed in percentages. Independent T- test was used to find the statistical significance of the association of age, dose of RAIT and ADR, whereas, chi-square test was done to find association of gender and adverse reactions following RAIT.
Results
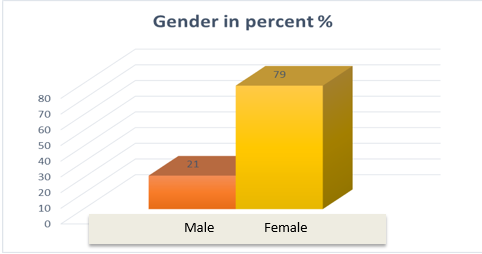
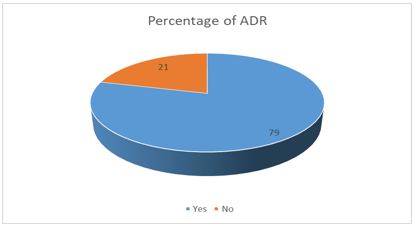
A total of 75 participants were included in this study of which 59 (79%) were females. The mean (standard deviation) of the age was 43(14). The dose of RAIT ranged from 30 mCi to 150 mCi, the mean(SD) of which is 94(24.6). The different types of ADRs were reported by 59 participants(79%).
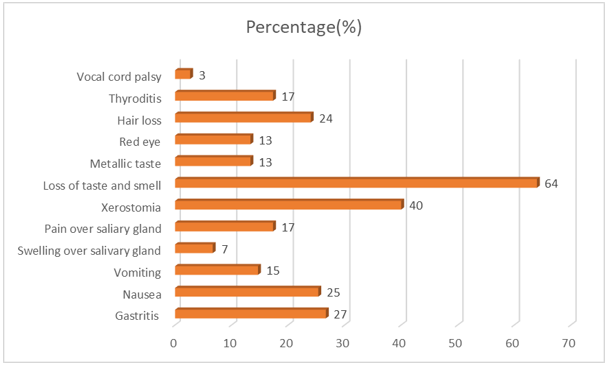
The most common adverse effect reported following RAIT was the loss of taste and smell in 48 participants (64%), followed by xerostomia in 30 patients (40%) and hair loss in 18 patients(24%) after one month of RAIT which subsided soon. These were not recorded in the follow-up visit.
A Chi-square test was done to find the significant association between gender and adverse effects due to RAIT. But the test revealed no significant association between them (p=0.686). An Independent t-test was done to find the significance between age, dose with adverse effects following RAIT. No significant association with age and presence of ADR due to RAIT (p value= 0.125). But dose of RAIT was found to have significant association with adverse events with increased dose producing ADR(p =0.008; p<0.05).
The loss of taste and smell, xerostomia and hair loss, which were the frequent ADRs were tested for their association with age, and dose with independent t-test and gender, but there was no association established (p=0.068;p=0.56;p=0.845 respectively). The complete blood counts were done before and after RAIT which was tested using paired t-test - showed a statistically significant decrease of all parameters including total count, neutrophils, lymphocytes, Hb, platelet count(p=0.00) with no clinically significant symptoms or signs.
Discussion
The disorders of the thyroid gland are common worldwide. There are multiple factors for the prevalence of thyroid gland disorders which include age, sex, and ethnic group. As thyroid hormones are an essential component for metabolism and survival, such disorders need to be identified and treated. Both dysregulations of hormone levels in the body and thyroid cancers are on the high.
In DTC, RAIT in the management of DTC has been employed post-surgery to ablate residual tissue and reduce recurrence.[24] Oral administration of I-131 is done 4 to 6 weeks post thyroidectomy.[24] In hyperthyroidism, the purpose of RAIT is to destroy enough thyroid tissue to make the patient euthyroid. The contraindications of RAIT are pregnancy, breastfeeding, and severely uncontrolled hyperthyroidism.[10]
The adverse effects of RAIT are usually mild - can be divided based on the duration - short term and long term adverse effects. Short-term adverse effects are those that occur within six months of RAIT; whereas long-term adverse effects are those occurring after six months. The short-term adverse effects are fairly common.[25] The study by Charalambous et al. revealed that the usual short-term adverse effects mainly include gastrointestinal discomfort, salivary dysfunction, neck pain and swelling, insomnia, and fatigue.[20] The long-term adverse include pulmonary fibrosis, secondary leukemia, early onset of menopause, and azoospermia rarely.[16]
Liyan Lu et. al. have reported in their study the short-term adverse effects of RAIT, which accounts for 67.5% of gastrointestinal adverse effects,[15] whereas the symptoms of gastritis, nausea, and vomiting were 27%, 25%, and 15% respectively in this study. It may also be due to the administration of corticosteroids post-RAIT. The gastrointestinal adverse effects in the study by Van Nostrand et al. also is 67%,[26] whereas it accounted for 34% in the study by Mohan et al.[27] Our study reported less number of gastrointestinal tract adverse reactions due to the use of proton pump inhibitors like pantoprazole and H2 blockers like ranitidine.
Apart from gastrointestinal adverse effects, the next important one was salivary gland dysfunction. Markitziu et al have reported a case of parotid gland enlargement even one-year post-surgery and RAIT.[28] Our study had 7% salivary gland swelling, 17% pain over the salivary gland, and 40% cases of xerostomia, which was comparable to the study of Van Nostrand et al.[26] Christof et al. in their 33% of cases of salivary gland dysfunction,[29] whereas Mohan et al found 47% cases of xerostomia alone.[27] The least cases obtained is 12.8%.[15] The next common adverse effect encountered was the loss of taste and smell which was 64% in our study. Compared to other studies (27%),[29] it was a bit high. 13% also complained of a metallic taste in the mouth.
The other adverse effects were thyroiditis (17%), hair loss (24%), and conjunctivitis (13%) in our study. The incidence of thyroiditis was low in our study owing to the low amount of residual tissue in post thyroidectomy patients compared to 47% in Liyan Lu et al. and 39% in Mohan et al. Benua et al. in their study found that 7.3% of patients reported having neck pain.[30]
Hair loss can occur as a part of hyperthyroidism, hypothyroidism, and dysfunction of parathyroid glands. [31] Also, hormone replacement can lead to a substantial amount of hair follicle loss, and hair may become brittle. [32] Hence, the causality of hair loss cannot be attributed as a whole to radioactive iodine therapy.
One of the rare adverse effects encountered was hoarseness of voice, less frequent and comparable to other studies.[15] Vocal cord paralysis was found to be unusually high (17%) i`n the study by Christof et al. Vocal cord paralysis can occur as part of the thyroid carcinoma or may follow injury to the vocal cord or nerves supplying it during surgery. Snyder et al. reported a case of vocal cord paresis in a 61-year-old female 1 week following RAIT suggesting the possible causality of RAIT.[33] Hoarseness of voice reported by our patients may or may not be due to vocal cord paralysis as we did not assess the vocal cord status or recurrent laryngeal nerve status pre-and post-treatment with RAI. The association between dose and adverse events could be established for the high dose of RAIT(p=0.008) with higher dose producing adverse effects.
Salivary gland dysfunction is a common adverse effect following radiotherapy to the head and neck. Parasympathomimetic drugs are used on and off for this purpose but are restricted due to their adverse effects. Pilocarpine is licensed for use in xerostomia induced by radiotherapy.[34] Another drug used is cevimeline for xerostomia secondary to Sjogren’s syndrome as used as a gargle by Y Takagi et al.[35] Due to these reasons, alternative non-pharmacological approaches are undertaken to prevent the destruction of the salivary gland and xerostomia following it. Christou A et al. have done a systematic review of 8 studies involving non-pharmacological measures including vitamin E, vitamin C, lemon candy, lemon juice, and parotid massage to minimize salivary gland damage.[36]
Hu T et al. demonstrated a significant dip in WBC, hemoglobin, and platelets following RAIT at higher doses which persisted for 6 months.[23] Molinaro E et al. produced similar results of significant lowering of WBC and platelets following a single 100mCi RAIT which lasted for 1 year.[37] Our study produced a statistically significant lowering of blood parameters (p=0.000). But clinically, this is not a significant fall for the patient to develop symptoms and consequences. Hester T et al. noted in their study that bone marrow function is not compromised in long term following RAIT, even though peripheral blood cell counts decreased.[38]
There were a few limitations to our studies. This was carried out on patients with thyroid carcinoma. The sample size was small. This limitation can be overcome by conducting the study in a larger sample size. Also, this study focused only on the short-term adverse effects of RAIT, and long-term adverse effects were not included due to the limited time period of this study.
Conclusion
This study was conducted to find out the pattern of short-term adverse effects of high-dose RAIT in patients with DTC. The adverse effects reported were mostly mild and self-limiting. The predominant ADR was the loss of taste and smell, xerostomia, fatigue, hair loss, and gastritis. The association of dose of RAIT and development of RAIT was established.
The proper monitoring of the adverse drug reactions and analysis is very important as it causes significant discomfort to the patients. The proper knowledge about the pattern of adverse effects is very important so that we can appraise the patient to take precautionary measures to reduce them. High-dose radioiodine therapy is a safe and non-invasive procedure.
Source of Funding
None.
Conflict of Interest
None.
References
- A Gessl, RL Gruber, AK Willer. Thyroid disorders. Handb Exp Pharmacol 2012. [Google Scholar]
- . Iodine-131 Medical Use. News-Medical.net2011 . [Google Scholar]
- A G Unnikrishnan, UV Menon. Thyroid disorders in India: An epidemiological perspective. Indian J Endocrinol Metab 2011. [Google Scholar]
- J H Lazarus, K Obuobie. Thyroid disorders-an update. Postgraduate Med J 2000. [Google Scholar]
- N R Caron, OH Clark. Papillary thyroid cancer. Curr Treat Options Oncol 2006. [Google Scholar]
- B Schmidbauer, K Menhart, D Hellwig, J Grosse. Differentiated Thyroid Cancer-Treatment: State of the Art. Int J Mol Sci 2017. [Google Scholar]
- W R Burns, MA Zeiger. Differentiated thyroid cancer. Semin Oncol 2010. [Google Scholar]
- S Padma, PS Sundaram. Radioiodine as an adjuvant therapy and its role in follow-up of differentiated thyroid cancer. J Cancer Res Ther 2016. [Google Scholar]
- . Radioactive Iodine (Radioiodine) Therapy for Thyroid Cancer. . [Google Scholar]
- M Mumtaz, LS Lin, KC Hui. Mohd Khir AS. Radioiodine I-131 For The Therapy Of Graves’ Disease. Malays J Med Sci 2009. [Google Scholar]
- . Sodium Iodide I 131 Capsules USP Diagnostic - Oral. . [Google Scholar]
- A Goolden, TR Fraser. Treatment of Thyrotoxicosis with Low Doses of Radioactive Iodine. BMJ 1969. [Google Scholar]
- S J Goldsmith. Radioactive Iodine Therapy of Differentiated Thyroid Carcinoma: Redesigning the Paradigm. Mol Imaging Radionucl Ther 2017. [Google Scholar]
- P Florenzano, F J Guarda, R Jaimovich, N Droppelmann, H González, JM Domínguez. Radioactive Iodine Administration Is Associated with Persistent Related Symptoms in Patients with Differentiated Thyroid Cancer. Int J Endocrinol 2016. [Google Scholar]
- L Lu, F Shan, W Li, H Lu. Short-Term Side Effects after Radioiodine Treatment in Patients with Differentiated Thyroid Cancer. Biomed Res Int 2016. [Google Scholar]
- B Bader. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 1998. [Google Scholar]
- M Nies, A Cantineau, E Arts, MH Van Den Berg, F V Leeuwen, M Kobold. Long-Term Effects of Radioiodine Treatment on Female Fertility in Survivors of Childhood Differentiated Thyroid Carcinoma. Thyroid . 2020. [Google Scholar]
- A F Esfahani, A E Ardekani, B Fallahi, P F Esfahani, D Beiki, A Hassanzadeh-Rad. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun 2014. [Google Scholar]
- S K Gill. Radiation Induced Salivary Gland Damage: Review on Pathogenesis and Management. Res Rev J Dent Sci 2016. [Google Scholar]
- A Charalambous. Seeking optimal management for radioactive iodine therapy-induced adverse effects. Asia Pac J Oncol Nurs 2020. [Google Scholar]
- N Beslic, S Licina, A Sadija, R Milardovic. Incidence of Hypothyreoidism after Radioactive Iodine-I131 Treatment in Dependance of Hyperthyreoidism Etiology and Therapy Dose. Med Arch 2017. [Google Scholar]
- R K Badam. Assessment of Salivary Gland Function Using Salivary Scintigraphy in Pre and Post Radioactive Iodine Therapy in Diagnosed Thyroid Carcinoma Patients. J Clin Diagn Res 2016. [Google Scholar]
- T Hu, Z Meng, G Zhang, Q Jia, J Tan, W Zheng. Influence of the first radioactive iodine ablation on peripheral complete blood count in patients with differentiated thyroid cancer: . Medicine 2016. [Google Scholar]
- N S Andresen, JM Buatti, HH Tewfik, NA Pagedar, CM Anderson, JM Watkins. Radioiodine Ablation following Thyroidectomy for Differentiated Thyroid Cancer: Literature Review of Utility, Dose, and Toxicity. Eur Thyroid J 2017. [Google Scholar]
- W Y Lin, YY Shen, SJ Wang. Short-term hazards of low-dose radioiodine ablation therapy in postsurgical thyroid cancer patients. Clin Nuclear Med 1996. [Google Scholar]
- D Nostrand, J Neutze, F Atkins. Side effects of “rational dose” iodine-131 therapy for metastatic well-differentiated thyroid carcinoma. Soc Nuclear Med 1986. [Google Scholar]
- S Mohan, G Agnihotri. Detriments of radioactive Iodine 131 in managing thyroid carcinoma - a retrospective study with review of the current scenario. 2016. [Google Scholar]
- A Markitziu, J Lustmann, B Uzieli, Y Krausz, R Chisin. Salivary and lacrimal gland involvement in a patient who had undergone a thyroidectomy and was treated with radioiodine for thyroid cancer. Oral Surg Oral Med Oral Pathol 1993. [Google Scholar]
- C Alexander, JB Bader, A Schaefer, C Finke, CM Kirsch. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 1998. [Google Scholar]
- R Benua, NR Cicale, M Sonenberg, RW Rawson. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. . Am J Roentgenol 1962. [Google Scholar]
- M Vincent, K Yogiraj. A Descriptive Study of Alopecia Patterns and their Relation to Thyroid Dysfunction. Int J Trichol 2013. [Google Scholar]
- K J Kumar, MS Kumar, TS Kumar, A Chavan. Diffuse scalp hair loss due to levothyroxine overdose. Indian Dermatol Online J 2015. [Google Scholar]
- S Snyder. Vocal Cord Paralysis after Radioiodine Therapy. J Nuclear Med 1978. [Google Scholar]
- A Davies, K Shorthose. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database Syst Rev 2007. [Google Scholar]
- Y Takagi, Y Kimura, T Nakamura. Cevimeline gargle for the treatment of xerostomia in patients with Sjögren’s syndrome.. Ann Rheumatic Dis 2004. [Google Scholar]
- A Christou, E Papastavrou, A Merkouris, S Frangos, P Tamana, A Charalambous. Clinical Studies of Nonpharmacological Methods to Minimize Salivary Gland Damage after Radioiodine Therapy of Differentiated Thyroid Carcinoma: Systematic Review. Evid Based Complement Alternat Med. 2016. [Google Scholar]
- E Molinaro, R Leboeuf, B Shue, AJ Martorella, M Fleisher, S Larson. Mild decreases in white blood cell and platelet counts are present one year after radioactive iodine remnant ablation. Thyroid 2009. [Google Scholar]
- H T Prinsen, K Hesselink, EN Brouwers, A H Plukker, J Sluiter, W J Van Der Horst-Schrivers. Bone Marrow Function After 131I Therapy in Patients With Differentiated Thyroid Carcinoma. J Clin Endocrinol Metab 2015. [Google Scholar]
How to Cite This Article
Vancouver
George A, Y A, P H, Kumari A. High dose radioactive iodine therapy and its short term adverse effects [Internet]. J Community Health Manag. 2025 [cited 2025 Sep 08];9(3):155-160. Available from: https://doi.org/10.18231/j.jchm.2022.030
APA
George, A., Y, A., P, H., Kumari, A. (2025). High dose radioactive iodine therapy and its short term adverse effects. J Community Health Manag, 9(3), 155-160. https://doi.org/10.18231/j.jchm.2022.030
MLA
George, Ancy, Y, Annapurna, P, Harilal, Kumari, Anila. "High dose radioactive iodine therapy and its short term adverse effects." J Community Health Manag, vol. 9, no. 3, 2025, pp. 155-160. https://doi.org/10.18231/j.jchm.2022.030
Chicago
George, A., Y, A., P, H., Kumari, A.. "High dose radioactive iodine therapy and its short term adverse effects." J Community Health Manag 9, no. 3 (2025): 155-160. https://doi.org/10.18231/j.jchm.2022.030
