Introduction
India began administration of COVID-19 vaccines on 16 January 2021. As of 11 May 2021, India has administered 175,235,991 doses overall, including first and second doses of the currently approved vaccines.1 Reliable quantification of the antibody response to SARS-CoV-2 vaccination is highly relevant for identifying possible vaccine efficiency and estimating the time of protection. The use of accessible, automated, high-throughput SARS-CoV-2 serology testing can aid in driving effective vaccine strategy by helping confirm efficacy and promote public health. Healthcare Professionals were the first group of beneficiaries of this vaccine. Therefore, we aimed to evaluate the neutralizing [anti-RBD] antibody responses induced by COVID-19 vaccines after first and booster dose in healthcare professionals.
Materials and Methods
69 healthcare professionals [HCPs] were enrolled for the evaluation study. The COVISHILED vaccine developed by AstraZeneca, University of Oxford and manufactured and distributed in India by the Serum Institute of India was administered to all the participants. Study spanned across 68 days and during this time span all participants were evaluated for the levels of neutralizing IgG antibodies against SARS-CoV-2 for 3 times. 1st evaluation of all participants to detect levels neutralizing IgG antibodies was conducted just before 1st vaccine dose. This helped to baseline neutralizing antibody levels amongst participants and record associated clinical history of previously diagnosed COVID-19 infection. Sequentially all participants were evaluated to detect levels of neutralizing IgG antibodies on 2 occasions: on approximately 27th day and on 68th day from baselining & first vaccine dose to assess change in levels of neutralizing IgG antibodies post vaccination.
Neutralizing antibodies are critical in the fight against COVID-19 because they defend cells from infection by the virus. Multiple studies indicate a primary role for neutralizing antibodies that target the spike protein of SARS-CoV-2. The antibodies against S1-RBD epitopes estimated to comprise ~90% of neutralizing activity. 2, 3, 4, 5
We used the US FDA Emergency Use Authorized [EUA], Atellica Solution SARS-CoV-2 IgG assay that detects anti S1-RBD antibodies including neutralizing IgG against SARS-CoV-2
As per kit insert of the assay, specimens with anti-RBD level ≥1 was termed reactive or sero-converted. Specimens that crossed measuring range of the assay were assayed using recommended dilution protocol using recommended dilution buffer as per package insert.
Results
When tested for levels neutralizing IgG antibodies for base lining, 18 out of 69 healthcare professionals [HCPs] were sero-converted showing detectable levels of IgG antibodies against SARS-CoV-2. Out of these 18 participants, 6 participants had clinical history of previously diagnosed COVID-19 infection and 12 participants were assumed to have suffered supposedly asymptomatic COVID-19 infection leading to sero-conversion. These 18 participants were monitored as separate cohort to study impact of clinically diagnosed as well as asymptomatic COVID-19 infection on production of neutralizing antibodies post vaccination.
After excluding 18 participants listed above the investigational cohort of 51 participants was selected to investigate neutralizing [anti-RBD] antibody responses induced by COVID-19 vaccine.
On approximately 27th day from first vaccine dose, 45 out of 51 (88.2%) participants in investigational cohort were sero-converted for anti-RBD antibodies [Figure 1]. Out of six participants that were yet to sero-convert, five demonstrated increased level of neutralizing [anti-RBD] antibodies but did not cross the reactivity threshold to confirm presence of neutralizing [anti-RBD] antibodies.
On approximately 68thday from second vaccine dose, 49 out of 51(96%) participants in investigational cohort were sero-converted for anti-RBD antibodies [Figure 1]. Four additional participants sero-converted post booster dose This amounts for sizable population [96.0%] that produced neutralizing [anti-RBD] antibodies post vaccination. Two participants continued to remain non-reactive.
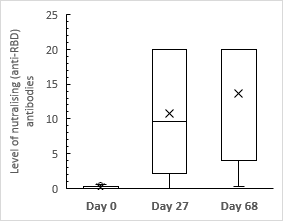
In investigational cohort the median level of neutralizing [anti-RBD] antibodies on approximately 68thday [≥20.0] reached 1000 folds higher than that observed during baselining on Day 0 [0.02] [Figure 2].
Figure 2
Median levels of neutralizing [anti-RBD] antibodies in investigational cohort at each evaluation stage
17 out of 26 participants in investigational cohort who completed two-dose vaccine schedules had achieved outraising [anti-RBD] levels which would have been high enough to qualify for high titer convalescent plasma donation.6 [Figure 3]
Figure 3
Comparing levels of neutralizing antibody levels of participants in investigational cohort against the test cut-off set as per amended EUA of the US FDA

Eight participants from investigational cohort were diagnosed with COVID-19 infection with positive RT-PCR results after administration of booster dose. Investigators did not have further access to the reported symptoms, severity, clinical findings, and duration of the infection.
18 participants that had seroconverted on Day 0 during baselining achieved high levels [median: ≥20] of neutralizing [anti-RBD] antibodies after a single vaccine dose. This was found to be at-par with the median levels reported by investigational cohort on 70th day post booster dose. [Figure 4]
Figure 4
Median levels of neutralizing [anti-RBD] antibodies in cohort demonstrating seroconversion prior to vaccination at each evaluation stage
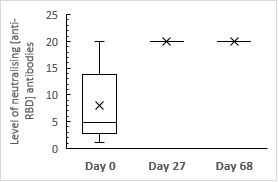
All the 18 participants achieved outraising [anti-RBD] levels which would have been high enough to qualify for convalescent plasma donation. During evaluation on approximately 68thday which was after administration of the booster dose, all the 18 participants continued to demonstrate equivalent level anti RBD antibodies [≥20.0] compared with those observed on approximately 27th day continuing to qualify as high titer convalescent plasma donation.6
Discussion
SARS CoV 2 infection has played a havoc globally in the last one year causing significant morbidity and mortality. Infection as well as vaccination has been shown to provide some degree of protection to individuals.
We reported 96.07% seroconversion rate in investigational cohort that had completed 2 dose regime of COVISHILED vaccine. In 18 participants with previous SARS-CoV-2 seroconversion, a single dose of the COVISHILED vaccine can induce high index values, mirroring findings from a recent HCW study.7 It has been shown in some studies that infected patients have about 89% protection from reinfection and different vaccines have been shown to confer about 50-95% protection.8 However, it is still unclear whether this immunity is long-lasting or will gradually wane over a period of time.9, 10, 11 Moreover, emergence of new variant Strains of the virus is a cause of concern as these may be able to escape the immunity provided by natural infection as well as by vaccination.12
The major challenge at present to assess the immunity provided by natural infection and post vaccination is the lack of standardized assays for detecting Spike protein neutralizing IgG antibodies. The only available data currently is from phasic trials of different types of vaccines. However, it is estimated that T abd B cell memory may contribute to some degree of protection and that neutralizing IgG antibodies against spike protein have a definite role in conferring immunity. The studies analyzing the titre of antibodies post vaccination are very limited as the vaccination drive in India has started few months back but picked momentum only recently. Few studies conducted outside India have found half-life of approx. 65 days for antibody titres.13 In a study on 102 residents of a nursing home in France, 36 residents with history of previous Covid infection were found to be positive for S protein IgG while 49.2% showed good immune response after vaccination.14 In a study by Bradley et al.,15 the level of blocking antibodies were shown to be higher in recently infected group as compared to sero negative individuals. Our study correlates with this study.
Conclusion
This is the first data of serological responses to COVID-19 vaccines in IBD patients with detailed analysis of antibodies RBD/spike proteins represented amongst HCPs from central India. The study is limited to our single centre experience and involves a small cohort representing only a section of population [HCPs].
Despite these limitations, our results clearly highlight the benefits of getting vaccinated against COVID-19 and thereby building adaptive immunity via vaccine induced neutralizing antibodies. Study also demonstrates that the level of neutralizing antibodies produced may vary due to several contributing factors and hence periodic monitoring of neutralizing antibodies before and post vaccination can help evaluate adaptive immune response induced by specific individual in response to vaccine administered.
Our study also highlights the need for future studies including more sections of common population & also earlier time points and longitudinal studies to guide optimal dosing regimens and/or vaccine choice in Indian population.


