Introduction
The COVID-19 pandemic has raged on for about a year. In India, 10,880,603 individuals were infected as on 12th February 2021, and over 7 million vaccinated since the vaccination drives began on 16th January 2021.1, 2, 3 Experts suggest that achieving herd immunity, also known as community immunity, might be a good way to break the chain of transmission of COVID-19 infection. Vaccines are the safest way to achieve acquired herd immunity. Cross-sectional population seroprevalence (the percentage of the population with serum containing antibodies that recognize the virus) data is crucial to plan vaccination strategies to cover the most susceptible sub-populations beyond the prioritized groups. Notably, a seroprevalence data based on measurement of clinically relevant neutralizing antibodies to SARS-CoV-2 infection, gives a legitimate estimate of the extent of immunity for baselining a population. Vaccination prioritization and policy making for public health promotion can be effectively achieved on the basis of periodic neutralizing antibodies serosurveillance data.
Objectives
This study examines the cross-sectional seroprevalence of anti-SARS-CoV-2 neutralizing IgG antibodies in 1561 individuals from city of Indore in India, using first and by far the only SARS-CoV-2 IgG assay approved under EUA by the USFDA for semiquantitative measurements. Disparate population groups of all ages and both sexes, including asymptomatic/ symptomatic, pediatric, geriatric, those with or without PCR test results, were included in the study.
Materials and Methods
A cross-sectional retrospective study was conducted using the serum samples obtained from 1556 individuals at a commercial clinical laboratory in Indore city of Central India in a span of approximately 3 months between 7th September 2020 to 9th December 2020. Siemens Healthineers COV2G assay that detects neutralizing IgG against the S1-RBD (receptor binding domain of spike protein subunit 1 antigen was selected to be used in this study. Sera were collected by thorough aseptic technique in serum tubes for 1556 individuals to be screened for SARS-CoV-2 neutralizing IgG S1 RBD antibodies. Participants were asked to declare any COVID-19-associated symptoms and were grouped into symptomatic and asymptomatic categories. Details of diagnosis of previous SARS-CoV-2 infection by RT-PCR test were also noted. Participants were tested for seroconversion by the measurement of anti-SARS-CoV-2 S1 RBD IgG antibodies on Atellica Solution, and were categorized as seropositive if SARS-CoV-2 antibodies were detected at a threshold of ≥ 1.0 index value; or seronegative if SARS-CoV-2 antibodies were below the threshold. Previous validation work with this assay demonstrated a 100% sensitivity (14 days post-PCR) and 99.95% specificity.4
Results
We report an overall seroconversion rate of 33% across our cohort.
Both the genders recorded seropositivity similar to the overall population, with prevalence among females and males being 36% and 31% respectively.(Figure 1)
Females demonstrated slightly higher [+5%] sero positivity than males in screened population.
88% of study population was between 18-60 years of age, and recorded a seropositivity of 31%, very similar to that of study cohort. Pediatric population defined as less than 18 years of age, comprised 3% of the individuals studied, while elderly population comprised 9% individuals included (Figure 2). Both these groups recorded approximately 10% higher seropositivity in comparison to overall cohort, 40% in pediatric, and 43% in the elderly population. (Table 1)
Table 1
Showing demographic data of positive & negative antibody
|
Age Group |
Non Reactive |
Reactive |
Total Specimen Counts |
% of Total Population |
Sero Positivity % |
|
<18 |
27 |
18 |
45 |
3% |
40% |
|
18-60 |
937 |
428 |
1365 |
88% |
31% |
|
>60 |
83 |
63 |
146 |
9% |
43% |
|
Total |
1047 |
509 |
1556 |
Distribution of SARS-CoV-2 seropositivity in symptomatic and asymptomatic individuals was studied. Amongst screened population 13% individuals [n=215] recorded presence COVID-19 related symptoms before serology test. 50% of the symptomatic population demonstrated seropositivity. 30% of asymptomatic population also demonstrated seropositivity. (Figure 3)
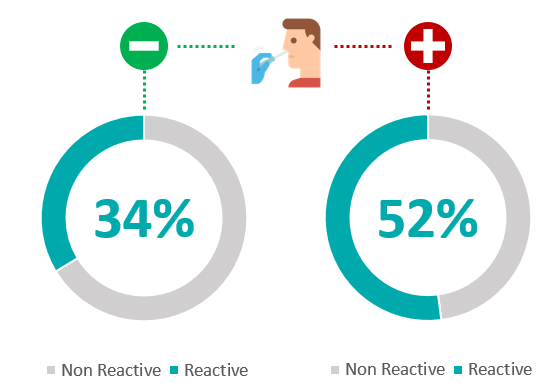
We also aimed at looking at distribution of SARS-CoV-2 seropositivity in RT-PCR positive and RT-PCR negative population. 390 individuals had recorded their PCR test results at or after antibody testing. Out of 194 individuals with positive RT-PCR test, 52% [n=101] were sero positive. And of the 196 individuals with negative RT-PCR test, 34% [n=66] were sero positive (Figure 4). Amongst 1000+ individuals that did not record any history of RT-PCR testing, 29% individuals [n=345] demonstrated sero-positivity. Thus, we found 52% and 34%, SARS-CoV-2 sero positivity in RT-PCR Positive and RT-PCR negative population, respectively. (Figure 4)
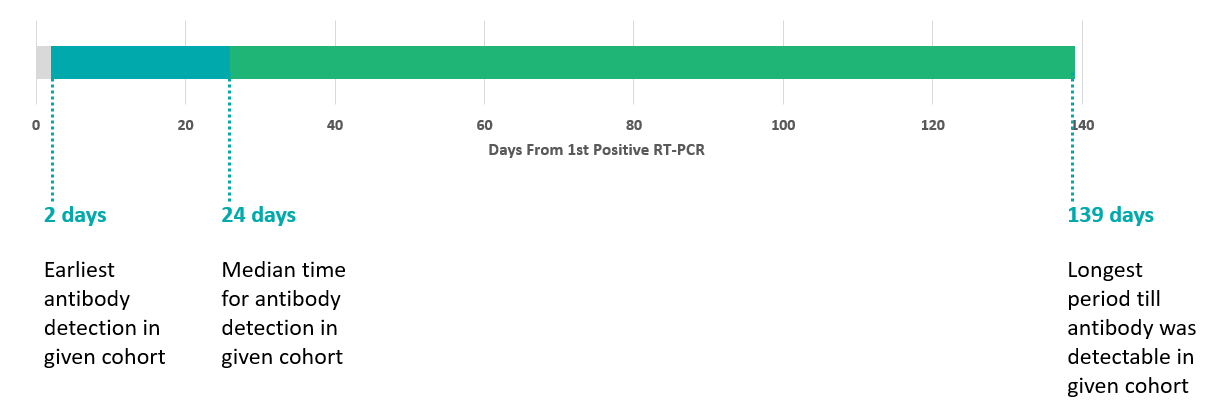
The timelines of antibody test reactivity from the first positive RT-PCR were noted. Detection timelines for anti S1-RBD IgG antibodies were available in 90 individuals that were RT-PCR positive, and the RT PCR was done before antibody assay. Number of days from first positive RT-PCR, ranged from earliest antibody detection in given cohort was 2 days, while the longest period till antibody was detectable in given cohort was 139 days. The median time for antibody detection in given cohort was 24 days. (Figure 5)
Discussion
Though numerous seroprevalence surveys have been done in the past, we believe that assessment of prevalence of neutralizing S1 RBD IgG antibodies to SARS-Cov-2 gives a true estimate of immunity. This is because anti-S antibodies, especially anti-RBD antibodies, can block the SARS-Cov-2 virus from recognizing and entering the target human cell, thus neutralizing the virus. In fact, a study authored by the US FDA, demonstrated that anti-RBD antibodies have the most potent neutralizing activity for the SARS-CoV-2 virus.5 In contrast, the viral nucleocapsid (N) protein does not bind the ACE2 receptor,6 so anti-N antibodies are not likely to have any viral neutralization activity.
Testing forSARS-CoV-2 IgG subclass antibodies serves as a surrogate endpoint for measuring vaccine response. The duration as well as extent of immune response can be monitored overtime. This is in setting of acquiring infection naturally or post vaccination. Noteworthy, Ig G antibodies to the S1RBD antigen have maximum neutralizing capability and are hence the most relevant antibodies to measure, when assessing for protection in a longitudinal study.
A sero survey report on Madhya Pradesh’s Indore made public on in August 2020 suggested that as many as 7.75 percent people of the city have developed antibodies against Covid-19.7 Our study reported a much higher seroconversion rate of 33%. As per ICMR’s latest serosurvey conducted in January 2021, 25% Indians now have antibodies against COVID-19. Prevalence reported in first survey was 0.70%, conducted in May-June2020, while that of second survey was 7% conducted in August 2020.8
Serological testing may be helpful for the identification of asymptomatic infections. Finding of 30% seropositivity in asymptomatic individuals in our study re-emphasizes the need of frequent serosurveys. Asymptomatic persons seem to account for approximately 40% to 45% of SARS-CoV-2 infections, and they can transmit the virus to others for an extended period, perhaps longer than 14 days.9 Because of the high risk for silent spread by asymptomatic persons, it is imperative that testing programs include those without symptoms. A meta-analysis published in December 2020, which included 13 studies involving 21,708 people, calculated the rate of asymptomatic presentation to be 17%.10
Most people who are infected with SARS-CoV-2 develop a humoral immune response within the first few weeks after infection. SARS-CoV-2 serology assays aid in diagnosis of individuals with suspected SARS-CoV-2 infection and as an aid in identifying patients with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. This may be due to natural infection or post vaccination. Zhao et al. found that median seroconversion time for IgG was day-14. The presence of antibodies was <40% among patients within 1-week since onset of symptoms, and rapidly rose to 79.8% since day-15 after onset of symptoms. In contrast, RNA detectability decreased from 66.7% in samples collected before day-7 to 45.5% during day 15-39.11 Thus, combining RNA and antibody detection significantly improved the sensitivity of pathogenic diagnosis for COVID-19, even in the early phase of 1 week from onset. Moreover, a higher titer of antibody was independently associated with a worse clinical classification. Our results too indicated more than 30% sero positivity in RT-PCR negative individuals, suggesting that combined use of the RT-PCR test and antibody assay may markedly improve the sensitivity of COVID-19 diagnosis.
In a study performed by Long, QX. et al., all patients achieved seroconversion of IgG or IgM within 20 days after symptom onset. The median day of seroconversion for both IgG and IgM was 13 days post symptom onset. Three types of seroconversion were observed: synchronous seroconversion of IgG and IgM, IgM seroconversion earlier than that of IgG and IgM seroconversion later than that of IgG. Our results showed antibody test reactivity from the first positive RT-PCR ranged from day 2 to day 139, mean being 24 days.
There are critical outstanding research needs today, most importantly, for defining a threshold for neutralizing antibody levels that correlates with immunity. Some thresholds have been defined for convalescent plasma donation, but no levels have been defined thus far for immunity claim. Filling in this gap with serology data will help us interpret antibody responses through widespread population testing. Current studies show a varied response to natural infection, and individuals probably respond differently across vaccines from different manufacturers. Additionally, there is a dearth of data today on duration of antibody response, and additional data across populations can help determine when a vaccine booster may be needed to assure sufficient neutralizing antibody presence remains long-term.
Conclusion
We provide evidence of SARS-CoV-2 seroconversion in individuals from Indore city with and without prior symptomatic illness. We report an overall seroconversion rate of 33% across our cohort which was significantly higher than previously reported seroconversion rate of 7.75% from the serosurveillance carried out by MGM and NCD jointly. Anti S1 RBD IgG antibodies were detectable in serum as early as 2 days and as long as 139 days from the first positive RT-PCR recorded. In light of further evidence of asymptomatic infection and seroconversion, the impact of mandatory population screening using serology assays should be thoroughly investigated.
We cannot exclude the possibility that this study underestimates SARS-CoV-2 infections as more recent infections that have not led to sero-conversion may have not been detected. Longitudinal studies involving serial sampling will be necessary to provide updated assessments of seroconversion and the longevity of antibody responses.
This serosurveillance study highlights the importance of measuring the prevalence of neutralizing antibody titers in general population across disparate groups. Along with other studies, it may subsequently guide policy making and developing vaccination strategies for those other than the prioritized groups. Insight into the current seroprevalence of SARS-CoV-2 antibodies within this cohort is of direct relevance as a reference point for future community serological surveys.